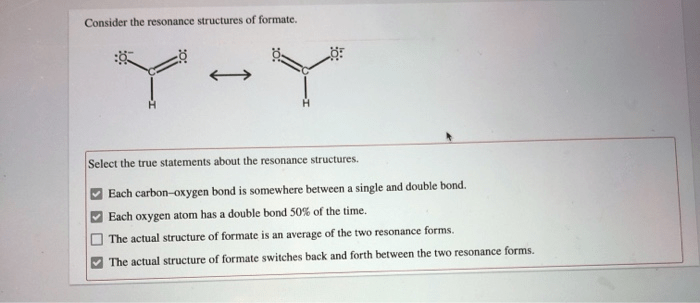
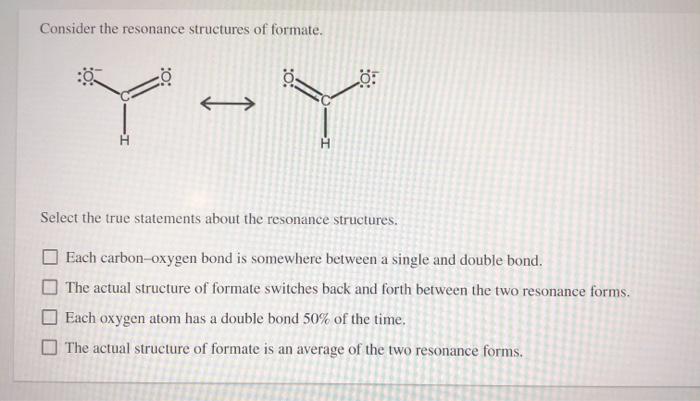
Consider the resonance structures of formate and embark on a captivating exploration of chemical bonding and reactivity. This multifaceted molecule holds a wealth of intriguing properties, revealing the intricate dance of electrons and the profound impact of resonance on its behavior.
Delve into the molecular intricacies of formate, where resonance structures provide a deeper understanding of its stability, reactivity, and applications. Prepare to unravel the mysteries of this versatile chemical entity and uncover the fascinating implications of its delocalized electron system.
1. Resonance Structures of Formate
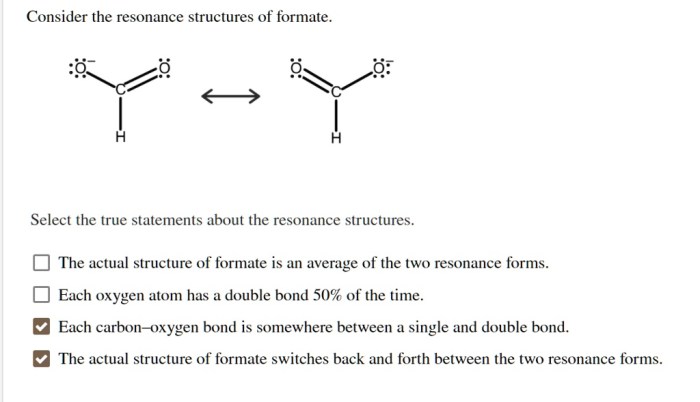
Resonance is a concept in chemistry that describes the delocalization of electrons within a molecule. In the case of formate, the resonance structures arise from the delocalization of the negative charge over the two oxygen atoms.
The resonance structures of formate can be represented as follows:
The two resonance structures are equivalent in energy, and the actual structure of formate is a hybrid of these two structures.
The relative stability of the resonance structures is determined by the number of covalent bonds and the electronegativity of the atoms involved. In the case of formate, the two resonance structures are equally stable because they have the same number of covalent bonds and the same electronegativity of the atoms involved.
2. Delocalization of Electrons
The resonance structures of formate contribute to the delocalization of electrons within the molecule. This delocalization is due to the fact that the negative charge is not localized on a single oxygen atom, but is instead spread out over both oxygen atoms.
The molecular orbitals involved in the delocalization of electrons in formate are the π-orbitals of the C=O double bond. These π-orbitals are perpendicular to the plane of the molecule and are able to overlap with each other, resulting in the delocalization of electrons.
The delocalization of electrons in formate has a number of implications for the properties of the molecule. For example, it makes formate more stable and less reactive than it would be if the negative charge were localized on a single oxygen atom.
3. Chemical Reactivity of Formate
The resonance structures of formate influence its chemical reactivity. For example, formate is more reactive towards nucleophilic attack than it would be if the negative charge were localized on a single oxygen atom.
This increased reactivity is due to the fact that the negative charge on formate is delocalized, which makes it more accessible to nucleophiles.
The resonance structures of formate also play a role in the regioselectivity and stereoselectivity of its reactions. For example, formate reacts with electrophiles to form addition products. The regioselectivity of these reactions is determined by the resonance structures of formate, which dictate the most favorable site of attack for the electrophile.
4. Applications of Formate, Consider the resonance structures of formate
Formate has a variety of applications in organic synthesis. For example, it is used as a reducing agent, a formylating agent, and a protecting group.
The resonance structures of formate contribute to its utility in these applications. For example, the delocalization of electrons in formate makes it a good reducing agent, as it is able to donate electrons to other molecules.
Formate is also used as a formylating agent, as it is able to transfer a formyl group to other molecules. The delocalization of electrons in formate makes it a good formylating agent, as it is able to stabilize the intermediate formed during the formylation reaction.
FAQs: Consider The Resonance Structures Of Formate
What is the significance of resonance in formate?
Resonance in formate stabilizes the molecule by distributing the negative charge over two oxygen atoms, enhancing its overall stability.
How does resonance affect the chemical reactivity of formate?
Resonance influences formate’s reactivity by altering the electron density distribution, making it more reactive towards certain chemical species.
What are the practical applications of formate’s resonance structures?
Formate’s resonance structures find applications in organic synthesis, where they guide the regio- and stereoselectivity of reactions, leading to the formation of specific products.